![[限時領取白皮書] Build your development and manufacturing path to IND](/upload/activity/activity_20230302122245_0.png)
[限時領取白皮書] Build your development and manufacturing path to IND
- 日 期:2023-03-02 ~ 2023-03-31
- 主辦單位:Lonza biologics
- 活動網址
活動介紹
Build your development and manufacturing path to IND
建立您新藥臨床試驗的開發及製造途徑
有彈性|量身打造|以里程碑為本
新藥臨床試驗(IND)的過程是複雜、具風險也耗費大量資源的。必須生成並解釋大量數據,以及同時進行許多步驟,但需利用先前獲得的結果以產生最好的成果。
關鍵考慮因素之一是最佳化您早期階段材料的使用,為此要建立符合您關鍵新藥臨床試驗里程碑的臨床前開發和製造途徑。您可以採循序漸進的方法並在不同的製造階段暫停,例如在non-GMP 製造、RCB、MCB 和/或毒理試驗,讓您讀出安全性和有效性研究或申請額外輪的資金。您可能希望在不中斷DNA-to-IND下採用加速的CMC途徑,並盡可能同時進行試驗。
點此領取
-3月限時領取!!
-於網站填寫姓名、公司等基本資料後即可下載
活動議程
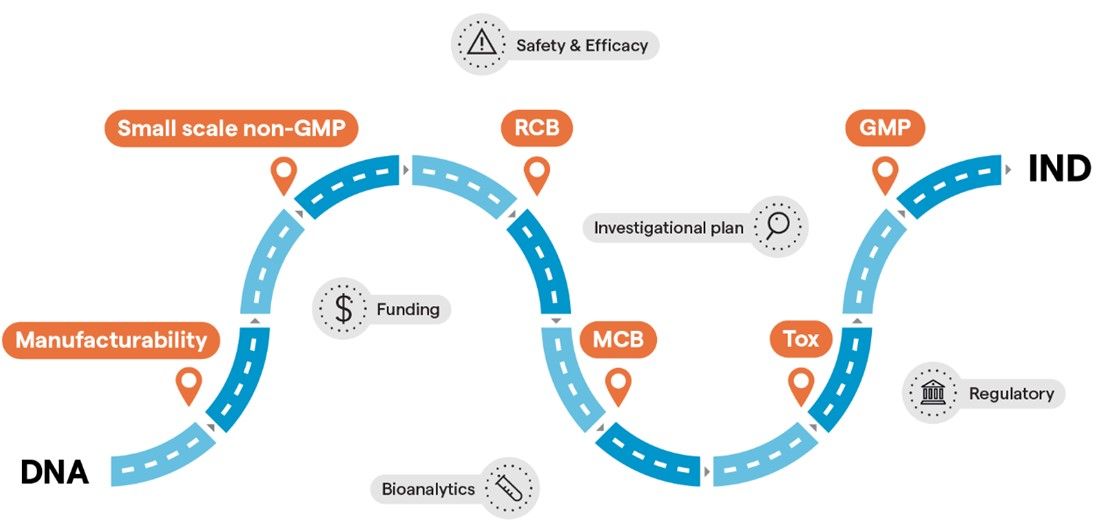
A few examples of how your path can look
In silico and in vitro manufacturability assessment
Vector construction
From MCB to GMP drug substance or drug product
From DNA to RCB or MCB
From DNA to tox drug substance or drug product
From DNA to IND-enabling CMC activities
Lonza is the preferred global partner to the pharmaceutical, biotech and nutrition markets. We focus on enabling treatments that prevent illness and support healthier lifestyles. We optimize scientific innovation and manufacturing technology to enable our customers to serve their patients and consumers.
We provide a wide range of services and products from early phase discovery to custom development and manufacturing of active pharmaceutical ingredients to innovative dosage forms for the pharma and consumer health and nutrition industries. Our scale and resources mean we can provide a one-stop solution for our customers to help people get well, feel well, and stay well. In 2021, we supported more than 780 preclinical and clinical small and large molecules, more than 245 commercial small and large molecules and produced around 250 billion capsules.




