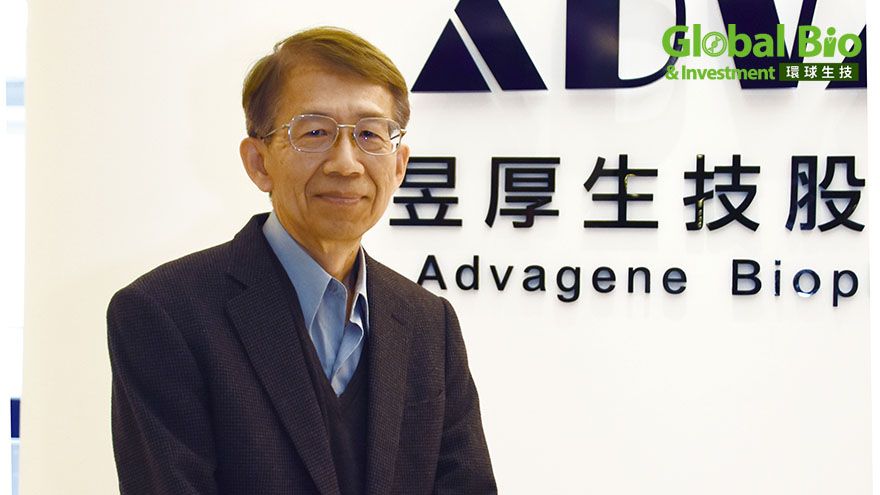
In October 2023, Advagene Biopharma’s nasal COVID-19 treatment AD17002-SC achieved impressive results in a Phase II trial, even passing the highly selective first round of evaluation by the U.S. Biomedical Advanced Research and Development Authority (BARDA). Nasal sprays are gaining recognition as a simpler, safer alternative for global pandemic preparedness.
Licensed from Taiwan’s Development Center for Biotechnology (DCB), LTh(αK) is a rare gem in the field of nasal spray protein drugs. This innovative modality could revolutionize vaccine adjuvants and respiratory treatments, creating new clinical and regulatory benchmarks worldwide.
Behind this success story is Dr. Hsu, the driving force who turned an early-stage discovery into a beacon of hope for patients everywhere.
Follow #Globalbio for more on Taiwan’s medical innovations!
#NasalSprayInnovation #BiotechBreakthroughs #GlobalHealthcare #VaccineRevolution #TaiwanExcellence