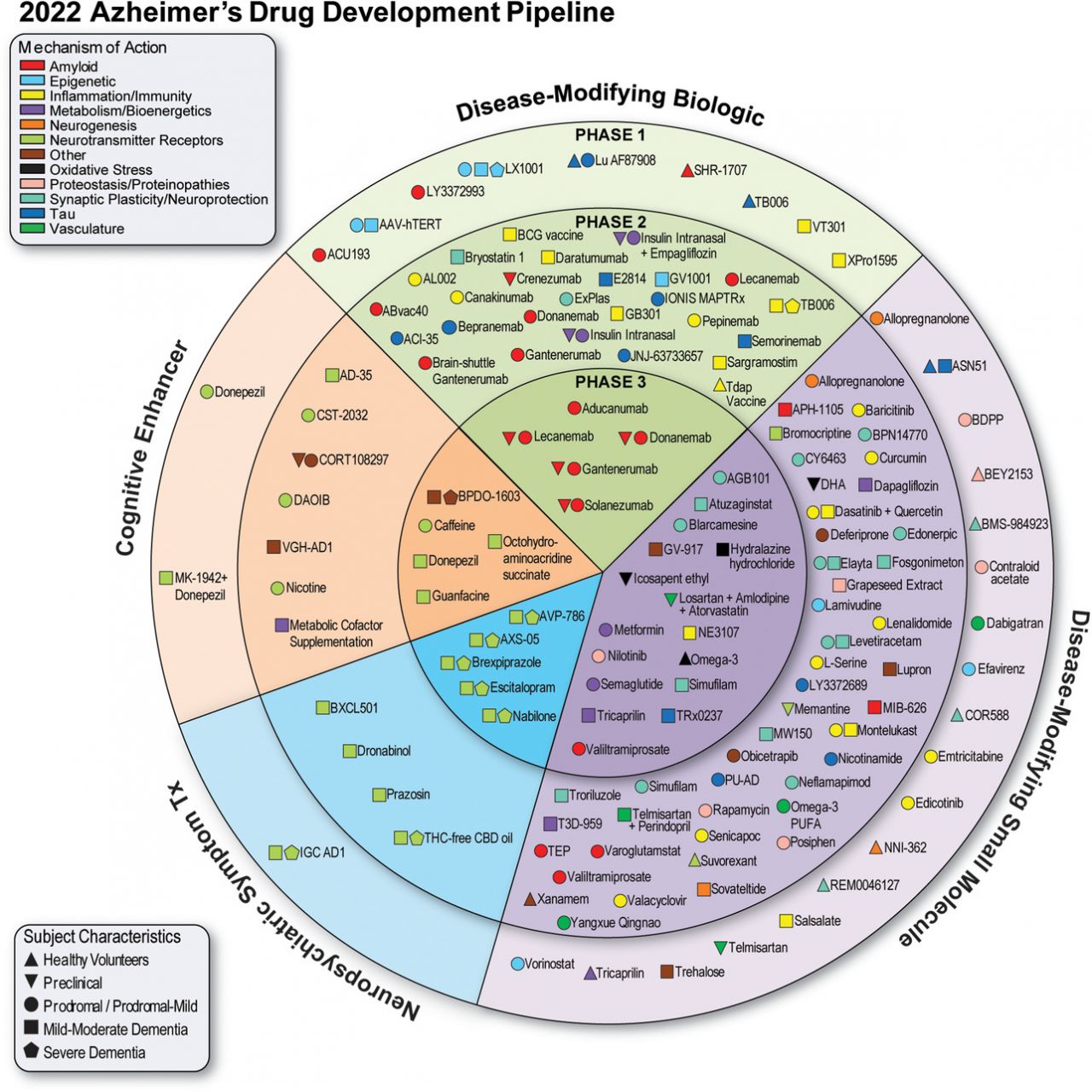
就藥物的目標分類,疾病緩解治療(Disease-modifying therapy, DMT)占大多數(83.2%),其餘則是針對症狀的治療,包括認知增強治療(9.8)和神經精神病學治療(6.9%)。在疾病緩解治療中,有34%為生物製劑,66%為小分子藥物。
其中,有37%的藥物是已批准用於其他適應症的老藥新用。
所有臨床試驗中,有50%為生物製藥公司贊助,而臨床三期試驗的比例則來到68%。若全部的臨床試驗都招募到足夠的受試者,總共會有5萬人參與。
在臨床三期試驗階段,共有31個藥物和47項試驗;在臨床二期試驗階段,共有82個藥物和94項試驗;在臨床一期試驗階段,共有30個藥物和31項試驗。以下分別就臨床一、二、三期簡述代表性的藥物。
圖片來源:https://doi.org/10.1002/trc2.12295
臨床三期試驗
除了已獲得批准但極具爭議的Aduhelm (aducanumab),百健(Biogen)和衛采(Eisai)共同研發的另一款藥物lecanemab,以及羅氏(Roche)子公司基因泰克(Genentech)的gantenerumab,和禮來(Eli Lilly)的donanemab,這三種藥物都是針對大腦中澱粉樣蛋白(Aβ)斑塊的單株抗體,預計在明年發布關鍵的臨床數據。
來自諾和諾德(Novo Nordisk)的第二型糖尿病藥物和減重藥物semaglutide,為GLP-1促效劑,則希望藉由改善神經細胞、發炎和血管健康,從而減緩阿茲海默症疾病的進展。Semaglutide有兩項三期研究,預計於2026年4月完成。
值得注意的還有Cassava Sciences的simufilam,該公司受到指控在早期的臨床前研究中操縱數據,調查過程還因此衍生案外案,一篇受引用超過兩千次的2006年《Nature》論文,遭踢爆疑似造假的醜聞。目前simufilam有兩項三期研究正在進行中,預計完成日期為2023年10月和2024年6月。
Quince Therapeutics (先前名為Cortexyme)的atuzaginstat,旨在減少牙齦卟啉單胞菌(Porphyromonas gingivalis)感染,該菌被認為與認知能力下降有關。然而,該療法的未來仍懸而未決,因為Atuzaginstat在2021年11月未達成一項臨床2/3期研究的主要終點,又在今年1月底被FDA臨床擱置;Cortexyme在更名為Quince後,該公司正在四處尋求收購或對外授權其神經科學和抗病毒資產。
臨床二期試驗
比利時跨國藥廠優時比(UCB Pharma)的bepranemab,針對的是另一種在大腦中積聚和纏結的蛋白質—tau蛋白,目前正在進行臨床二期試驗,治療患有輕度認知功能障礙(MCI)或輕度阿茲海默症的患者。
瑞士藥廠AC Immune和楊森(Janssen)同樣也在tau上押注了一些籌碼,雙方共同開發的阿茲海默症疫苗ACI-35,誘導免疫系統產生攻擊tau蛋白的抗體,其二期試驗預計在2023年10月完成。
Athira Pharma的fosgonimeton則是一種肝臟生長因子受體促效劑,在6月公布的二期試驗結果中,在主要試驗終點失敗,未能提高工作記憶和記憶處理速度。儘管如此,Athira仍指出該研究的亞組分析結果「非常令人鼓舞」,預計將據此調整臨床三期試驗的設計。
臨床一期試驗
禮來的Remternetug (LY3372993)為donanemab的後續產品,禮來稱為「次世代抗澱粉樣蛋白抗體」,預計於明年9月完成第一期臨床試驗。
乘著百健/衛采的Aduhelm在2021年6月獲得批准的浪潮,Acumen Pharmaceuticals在當時IPO籌集了1.6億美元,用於推動ACU193的臨床試驗。該藥物最初由Acumen與默沙東(MSD)在2003年到2011年間共同開發,為針對Aβ寡聚體的單株抗體。
參考資料:https://www.fiercebiotech.com/biotech/beyond-headlines-and-amyloid-alzheimers-pipeline-chugs-along
研究:
https://alz-journals.onlinelibrary.wiley.com/doi/full/10.1002/trc2.12295?af=R
(編譯/劉馨香)
【臨床三期試驗列表】
| Agent | Sponsor | CADRO mechanism class | Mechanism of action | Therapeutic purpose | Estimated end date |
|---|---|---|---|---|---|
| Aducanumab | Biogen | Amyloid | Monoclonal antibody directed at Aβ plaques and oligomers | DMT | 2023/10/1 |
| AGB101 (low-dose levetiracetam) | AgeneBio, NIA | Synaptic Plasticity/Neuroprotection | SV2A modulator; to reduce Aβ-induced neuronal hyperactivity | DMT | 2022/12/1 |
| Atuzaginstat (COR388) | Cortexyme | Synaptic Plasticity/Neuroprotection | Bacterial protease inhibitor targeting gingipain produced by P. gingivalis to reduce neuroinflammation and hippocampal degeneration | DMT | 2022/12/1 |
| AVP-786 | Avanir | Neurotransmitter receptors | Sigma 1 receptor agonist; NMDA receptor antagonist | Neuropsychiatric symptoms agent (agitation) | 2022/7/1 |
| Avanir | 2023/10/1 | ||||
| Avanir | 2024/12/1 | ||||
| Avanir | 2024/12/1 | ||||
| AXS-05 | Axsome therapeutics | Neurotransmitter receptors | NMDA receptor antagonist; combination of dextromethorphan and bupropion | Neuropsychiatric symptoms agent (agitation) | 2022/12/1 |
| Axsome therapeutics | 2023/6/1 | ||||
| Blarcamesine (ANAVEX2-73) | Anavex life sciences | Synaptic plasticity/neuroprotection | Sigma-1 receptor agonist, M2 autoreceptor antagonist; to ameliorate oxidative stress, protein misfolding, mitochondrial dysfunction, and inflammation | DMT | 2022/6/1 |
| Anavex life sciences | 2024/6/1 | ||||
| BPDO-1603 | Hyundai pharmaceutical | Undisclosed | Undisclosed | Cognitive enhancer | 2023/3/1 |
| Brexpiprazole | Otsuka | Neurotransmitter receptors | Atypical antipsychotic; D2 receptor partial agonist; serotonin-dopamine modulator | Neuropsychiatric symptoms agent (agitation) | 2022/8/1 |
| Otsuka | 2022/7/1 | ||||
| Otsuka | 2022/4/1 | ||||
| Caffeine | University Hospital, Lille | Neurotransmitter receptors | Adenosine antagonist; non-specific phosphodiesterase inhibitor | Cognitive enhancer | 2024/11/1 |
| Donanemab | Eli Lilly | Amyloid | Monoclonal antibody specific for pyroglutamate form of Aβ | DMT | 2025/8/1 |
| Eli Lilly | 2027/11/1 | ||||
| Donanemab & Aducanumab | Eli Lilly | Amyloid | Monoclonal antibody specific for pyroglutamate form of Aβ (donanemab); monoclonal antibody directed at plaques and oligomers (aducanumab); given in separate arms of the trial | DMT | 2023/6/1 |
| Donepezil | Assistance Publique – Hôpitaux de Paris | Neurotransmitter receptors | Acetylcholinesterase inhibitor | Cognitive enhancer | 2024/7/1 |
| Escitalopram | Johns Hopkins University, NIA | Neurotransmitter receptors | Selective serotonin reuptake inhibitor | Neuropsychiatric symptoms agent (agitation) | 2022/8/1 |
| Gantenerumab | Roche | Amyloid | Monoclonal antibody directed at Aβ plaques and oligomers | DMT | 2026/10/1 |
| Roche | 2023/8/1 | ||||
| Roche | 2023/4/1 | ||||
| Roche | 2024/12/1 | ||||
| Gantenerumab & Solanezumab | Washington University, Eli Lilly, Roche, NIA, Alzheimer's Association | Amyloid | Monoclonal antibody directed at Aβ plaques and oligomers (gantenerumab); Monoclonal antibody directed at Aβ monomers (solanezumab); given in separate arms of the trial | DMT | 2022/7/1 |
| Guanfacine | Imperial College London, UK National Institute of Health Research | Neurotransmitter receptors | Alpha-2 adrenergic agonist | Cognitive enhancer | 2022/12/1 |
| GV-971 | Shanghai Greenvalley | Gut-brain axis | Algae-derived acidic oligosaccharides; changes microbiome to reduce peripheral and central inflammation | DMT | 2026/10/1 |
| Hydralazine | Shahid Sadoughi University, Iran | Oxidative stress | Free radical scavenger | DMT | 2023/12/1 |
| Icosapent ethyl (IPE) | VA Office of Research and Development, University of Wisconsin, Madison | Oxidative stress | Purified form of the omega-3 fatty acid EPA; to improve synaptic function and reduce inflammation | DMT | 2023/1/1 |
| Lecanemab (BAN2401) | Eisai, Biogen | Amyloid | Monoclonal antibody directed at Aβ protofibrils | DMT | 2024/8/1 |
| Eisai, Biogen, ACTC, NIA | 2027/10/1 | ||||
| Losartan & Amlodipine & Atorvastatin + exercise | University of Texas Southwestern | Vasculature | Angiotensin II receptor blocker (losartan), calcium channel blocker (amlodipine), cholesterol agent (atorvastatin) | DMT | 2022/1/1 |
| Metformin | Columbia University, NIA | Metabolism and bioenergetics | Insulin sensitizer to improve CNS glucose metabolism | DMT | 2025/4/1 |
| Nabilone | Sunnybrook Health Sciences Center, ADDF | Neurotransmitter receptors | Synthetic cannabinoid | Neuropsychiatric symptoms agent (agitation) | 2025/10/1 |
| NE3107 | Neurmedix | Inflammation | MAPK-1/3 inhibitor; reduces proinflammatory NFκB activation | DMT | 2023/1/1 |
| Nilotinib BE | KeifeRx | Proteostasis/Proteinopathies | Tyrosine kinase inhibitor; autophagy enhancer; promotes clearance of Aβ and tau | DMT | 2026/6/1 |
| Octohydro-aminoacridine Succinate | Shanghai Mental Health Center | Neurotransmitter receptors | Acetylcholinesterase inhibitor | Cognitive enhancer | 2021/2/1 |
| Omega-3 (DHA+EPA) | University Hospital, Toulouse | Oxidative stress | Antioxidant | DMT | 2023/12/1 |
| Semaglutide | Novo Nordisk | Metabolism and bioenergetics | GLP-1 agonist; reduces neuroinflammation and improves insulin signaling in the brain | DMT | 2026/4/1 |
| Novo Nordisk | 2026/4/1 | ||||
| Simufilam (PTI-125) | Cassava sciences | Synaptic Plasticity/Neuroprotection | Filamin A protein inhibitor; stabilizes amyloid-alpha-7 nicotinic receptor interaction | DMT | 2023/10/1 |
| Cassava sciences | 2024/6/1 | ||||
| Solanezumab | Eli Lilly, ATRI | Amyloid | Monoclonal antibody directed at Aβ monomers | DMT | 2023/6/1 |
| Tricaprilin | Cerecin | Metabolism and bioenergetics | Caprylic triglyceride; induces ketosis and improves mitochondrial and neuronal function | DMT | 2024/2/1 |
| TRx0237 | TauRx Therapeutics | Tau | Tau protein aggregation inhibitor | DMT | 2023/3/1 |
| Valiltramiprosate (ALZ-801) | Alzheon, NIA | Amyloid | Prodrug of tramiprosate; inhibits Aβ aggregation into toxic oligomers | DMT | 2024/5/1 |
編輯推薦
- 默沙東重返阿茲海默症戰場!砸11億美元攜手Cerevance尋新靶點
- 繞過消化系統!Corium輕度阿茲海默症經皮貼片 獲FDA批准、腸胃道副作用明顯降低
- 美研究揭阿茲海默症最早警訊:「大腦炎症、睡眠障礙」為風險指標
- 衛采專注阿茲海默症 關閉腫瘤藥子公司、裁員80人
- 拚核酸國產自主化!基米攜手法信諾開發阿茲海默症核酸新藥
- 牙周病菌促海馬迴發炎、Tau蛋白磷酸化!中國研究揭阿茲海默症威脅
- 阿茲海默療法新進展!衛采、百健第二款新藥FDA提上市申請、中國首款新藥全球3期臨床暫停
- 百健砍Aduhelm商化資源;Accelerated hTSC轉iPSC;FDA批准超音波骨折醫材、首個早期阿茲海默IVD;正揚生醫獲多獎;臺大醫高端腸病毒疫苗登Lancet
- 百健放棄進軍歐洲! 撤回阿茲海默藥Aduhelm上市申請
- 受Aduhelm批准正向影響 羅氏再啟阿茲海默症Gantenerumab大型研究









